4.2d
Plastics are polymers, but polymers don't have to be plastics. The way plastics are made is actually a way of imitating nature, which has created a huge number of polymers. Cellulose, the basic component of plant cell walls is a polymer, and so are all the proteins produced in your body and the proteins you eat. Another famous example of a polymer is DNA - the long molecule in the nuclei of your cells that carries all the genetic information about you.
People have been using natural polymers, including silk, wool, cotton, wood, and leather for centuries. These products inspired chemists to try to create synthetic counterparts, which they have done with amazing success.
People have been using natural polymers, including silk, wool, cotton, wood, and leather for centuries. These products inspired chemists to try to create synthetic counterparts, which they have done with amazing success.
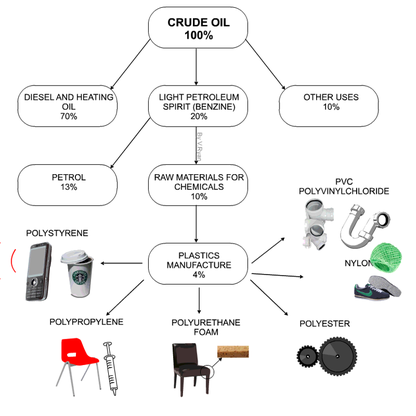
- Plastics are synthetic materials derived from organic (carbon-containing) compounds. The most common sources for carbon compounds are oil (petroleum) and natural gas.
- Plastics consist of polymers - long molecule chains - often mixed with other substances such as colouring agents and softeners.
- The properties of a particular plastic depend on what the polymer chains look like, how they are bonded to each other, and which additives have been introduced.
•Technically plastics are referred to as Polymers
•Petro-chemicals are the main ingredient for modern day plastics.
•Roughly 8% of oil production is due in plastics
•The raw material for plastics (mainly oil) is extracted in a country, exported to other countries where conversion to plastics takes place and these are re-exported at considerable added value. (Int Mind)
•Biomass – such as polylactic acid (PLA) resin which is made from corn or potato starch – is also biodegradable.
•Interesting note on compostability of PLA
•Petro-chemicals are the main ingredient for modern day plastics.
•Roughly 8% of oil production is due in plastics
•The raw material for plastics (mainly oil) is extracted in a country, exported to other countries where conversion to plastics takes place and these are re-exported at considerable added value. (Int Mind)
•Biomass – such as polylactic acid (PLA) resin which is made from corn or potato starch – is also biodegradable.
•Interesting note on compostability of PLA
There are 2 main types of plastics -
- Thermosets - Made from long chain molecules which bond together strongly when the plastics are made. This means that they cannot be re-melted. Thermosets are rigid and non-flexible even at high temperatures.
- Thermoplastics - Also made from long chain molecules which are entangled but not bonded together. This means that they can be melted and re-formed. (“Thermo” = heat, “plastic” = able to be formed)
Types of plastic
There are about 50 different groups of plastics, with hundreds of different varieties. All types of plastic are recyclable. To make sorting and thus recycling easier, the American Society of Plastics Industry developed a standard marking code to help consumers identify and sort the main types of plastic. These types and their most common uses are:
There are about 50 different groups of plastics, with hundreds of different varieties. All types of plastic are recyclable. To make sorting and thus recycling easier, the American Society of Plastics Industry developed a standard marking code to help consumers identify and sort the main types of plastic. These types and their most common uses are:
|
PET
Polyethylene terephthalate - Fizzy drink bottles and oven-ready meal trays. HDPE High-density polyethylene - Bottles for milk and washing-up liquids. PVC Polyvinyl chloride - Food trays, cling film, bottles for squash, mineral water and shampoo. LDPE Low density polyethylene - Carrier bags and bin liners. PP Polypropylene - Margarine tubs, microwaveable meal trays. PS Polystyrene - Yoghurt pots, foam meat or fish trays, hamburger boxes and egg cartons, vending cups, plastic cutlery, protective packaging for electronic goods and toys. OTHER Any other plastics that do not fall into any of the above categories. - An example is melamine, which is often used in plastic |
Covalent Bond
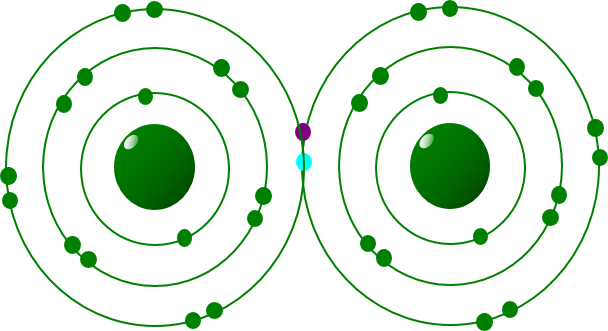
In a covalent bond the outer electrons of some atoms come close enough to overlap and are shared between the nuclei, forming a covalent bond. Each pair of electrons is called a covalent bond. Mention of sigma (σ), pi (π), double or triple bonds is not required. Covalent bonds are strong bonds and examples of primary bonds (as are metallic and ionic bonds).
In a Covalent bond the outer electrons of some atoms can come close enough to overlap and be shared between the nuclei, thereby forming a covalent bond.
In a covalent bond the outer electrons of some atoms come close enough to overlap and are shared between the nuclei, forming a covalent bond. Each pair of electrons is called a covalent bond. Mention of sigma (σ), pi (π), double or triple bonds is not required. Covalent bonds are strong bonds and examples of primary bonds (as are metallic and ionic bonds).
In a Covalent bond the outer electrons of some atoms can come close enough to overlap and be shared between the nuclei, thereby forming a covalent bond.
Secondary Bonds
Secondary bonds are weak forces of attraction between molecules that exist in substances such as water. Water is formed by polar covalent bonds (Polar Covalent bonds - electrons shared unequally, resulting in a partial negative/positive charge), which determine the arrangement of water molecules.
Secondary bonds are weak forces of attraction between molecules that exist in substances such as water. Water is formed by polar covalent bonds (Polar Covalent bonds - electrons shared unequally, resulting in a partial negative/positive charge), which determine the arrangement of water molecules.

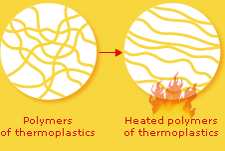
Thermoplastics are linear chain molecules with weak secondary bonds between the chains.
A thermoplastic is a plastic that melts to a liquid when heated and freezes to a brittle, very glassy state when cooled sufficiently. Most thermoplastics are high molecular weight polymers whose chains associate through weak Van der Waals forces (e.g. polyethylene); stronger dipole-dipole interactions and hydrogen bonding (nylon); or even stacking of aromatic rings (polystyrene).
Thermoplastic polymers differ from thermosetting polymers (Bakelite; vulcanized rubber) as they can, unlike thermosetting polymers, be reheated and remoulded. Many thermoplastic materials are addition polymers; e.g., vinyl chain-growth polymers such as polyethylene and polypropylene.
A thermoplastic is a plastic that melts to a liquid when heated and freezes to a brittle, very glassy state when cooled sufficiently. Most thermoplastics are high molecular weight polymers whose chains associate through weak Van der Waals forces (e.g. polyethylene); stronger dipole-dipole interactions and hydrogen bonding (nylon); or even stacking of aromatic rings (polystyrene).
Thermoplastic polymers differ from thermosetting polymers (Bakelite; vulcanized rubber) as they can, unlike thermosetting polymers, be reheated and remoulded. Many thermoplastic materials are addition polymers; e.g., vinyl chain-growth polymers such as polyethylene and polypropylene.
van der Waals Bond
The van der Waals bonds occur to some extent in all materials but are particularly important in plastics and polymers. These materials are made up of a long string molecules consisting of carbon atoms covalently bonded with other atoms. The covalent bonds within the molecules are very strong and rupture only under extreme conditions. The bonds between the molecules that allow sliding and rupture to occur are called van der Waals forces.
Deformation occurs in two ways:
The van der Waals bonds occur to some extent in all materials but are particularly important in plastics and polymers. These materials are made up of a long string molecules consisting of carbon atoms covalently bonded with other atoms. The covalent bonds within the molecules are very strong and rupture only under extreme conditions. The bonds between the molecules that allow sliding and rupture to occur are called van der Waals forces.
Deformation occurs in two ways:
- elastic, in which initially coiled chains are stretched and the material returns to its original size and shape when the load is removed.
- plastic, when at higher loads the secondary bonds between the chains weaken and allow the molecular chains to slide over each other, and the material does not return to its original size and shape when the load is removed. Creep and flow are important. No quantitative details are required.
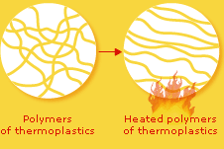
Thermoplastics have long, linear polymer chains that are only weakly chemically bonded, or connected, to each other.
When a thermoplastic object is heated, these bonds are easily broken, which makes the polymers able to glide past each other like strands of freshly cooked spaghetti. That's why thermoplastics can readily be remoulded.
The weak bonds between the polymers reform when the plastic object is cooled, which enable it to keep its new shape.
Thermoplastics are easy to recycle since they can be melted and reshaped into other products.
For example, a plastic bottle that contained a soft drink could be reformed into the fibres of a fleece jacket.
When a thermoplastic object is heated, these bonds are easily broken, which makes the polymers able to glide past each other like strands of freshly cooked spaghetti. That's why thermoplastics can readily be remoulded.
The weak bonds between the polymers reform when the plastic object is cooled, which enable it to keep its new shape.
Thermoplastics are easy to recycle since they can be melted and reshaped into other products.
For example, a plastic bottle that contained a soft drink could be reformed into the fibres of a fleece jacket.

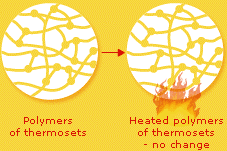
Thermosets are linear chain molecules with strong primary bonds between adjacent polymer chains. This gives thermosets a rigid 3D structure.
The linear chains are crosslinked - strongly chemically bonded. This prevents a thermoplastic object from being melted and reformed.
Just as a raw egg has the potential to become a boiled egg, a fried egg, and so on, thermosetting polymers have the potential to become all sorts of different objects.
Once an egg has been boiled, however, you can't make it into a fried egg. In the same way, once a thermosetting plastic object has been formed, it can't be remade into a different object.
Once an egg has been boiled, however, you can't make it into a fried egg. In the same way, once a thermosetting plastic object has been formed, it can't be remade into a different object.
|
Urea-formaldehyde, also known as urea-methanal, named so for its common synthesis pathway and overall structure, is a transparent thermosetting resin or plastic, made from urea and formaldehyde heated in the presence of a mild base such as ammonia or pyridine. These resins are used in adhesives, finishes, MDF, and moulded objects. Urea-formaldehyde resin's attributes include high tensile strength, flexural modulus and heat distortion temperature, low water absorption, mould, high surface hardness, elongation at break, and volume resistance. Urea formaldehyde was commonly used when producing electrical appliances casing i.e. desk lamps. It is now mostly replaced by melamine resin. Urea-formaldehyde foam insulation started being used in the 1950s. In the 1980s, concerns began to develop about the toxic formaldehyde vapour emitted in the curing process, as well as from the breakdown of old foam. Consequently, its use was discontinued. http://en.wikipedia.org/wiki/Urea-Formaldehyde |

Although PVC disposal is problematic, PVC is still widely used as a structural material, for example, in windows and for guttering and drainpipes.
Plastic facts in the UK:
Plastic disposal problems
Incineration:
Energy can be obtained from burning waste, but this is less energy than can be saved by recycling. In addition:
Plastics and the environment
There are environmental impacts from plastic production, plastic use and plastic waste disposal. Plastic containers are now lighter than they used to be, using less material, but our consumption of plastic is still set to rise. The building blocks of plastic, known as monomers, are made from oil and gas (plastic production uses 8% of the world oil production each year). To make the various polymers used by industry for various uses (for example, PET for plastic bottles, polystyrene, PVC) many chemicals are used which have not undergone a risk assessment.
Plastic facts in the UK:
- We produce 3 million tonnes of plastic every year;
- Households are the biggest producers of plastic waste;
- 60% of household waste comes from packaging;
- Over 60% of litter on beaches is plastic;
- More than 80% of all this plastic is used once and then thrown into landfill sites;
- Only 7% of plastic is recycled.
Plastic disposal problems
Incineration:
Energy can be obtained from burning waste, but this is less energy than can be saved by recycling. In addition:
- Incineration destroys valuable resources;
- Burning fossil fuels like coal, oil and gas is causing increasing levels of carbon dioxide in our atmosphere, leading to climate change. Incineration contributes to climate change, because when materials are burnt more fossil fuel energy is used to replace them through mining, manufacturing, and transportation around the world. Energy from burning waste is not renewable;
- Incinerators need a steady stream of waste to keep them going. This means there is no incentive to reduce waste or recycle it;
- Incineration causes pollution from air emissions and toxic ash;
- Incineration is worse for climate change than recycling because new products have to be made to
- Replace those destroyed;
- Incineration does not provide the thousands of new job opportunities that recycling does.
Plastics and the environment
There are environmental impacts from plastic production, plastic use and plastic waste disposal. Plastic containers are now lighter than they used to be, using less material, but our consumption of plastic is still set to rise. The building blocks of plastic, known as monomers, are made from oil and gas (plastic production uses 8% of the world oil production each year). To make the various polymers used by industry for various uses (for example, PET for plastic bottles, polystyrene, PVC) many chemicals are used which have not undergone a risk assessment.
|
Plastic disposal problems Landfill:
Friends of the Earth wants a ban on new landfill capacity until policies are in place to achieve the 60-80 per cent recycling rates achieved in other countries, because:
|

The PVC waste crisis (from Greenpeace http://archive.greenpeace.org/toxics/html/content/pvc3.html#recycle)
The world is facing a waste crisis from PVC. Short-life PVC products, disposed of within a few years, have caused serious PVC waste problems, especially when incinerated.
The average life span of the durable products -which make up more than half of PVC consumption - is around 34 years. Durable vinyl goods produced and sold since the 1960s - when the plastic boom began - are now just starting to enter the waste stream. We are only now seeing the first stages of an impending PVC waste mountain.
There are currently over 150 million tonnes of long-life PVC materials in existence globally, used mostly in the construction sector, which will constitute this waste mountain in coming decades. Taking into account the ongoing growth in production, by the year 2005 this amount will double and the world will have to deal with approximately 300 million tonnes of PVC starting to enter the waste stream. The amount of PVC waste arising in industrialised countries is already expected to grow faster than PVC production.
So what do we do with this waste? Is there a solution? Since PVC, like most plastics, does not biodegrade quickly, three primary options exist: bury it, incinerate it or recycle it.
PVC Recycling
In the late 1980s, PVC recycling was promoted by the vinyl industry in order to make PVC more acceptable to the public and to prevent government action to limit PVC production and use. As a result, the general public and decision-makers are now accepting recycling as a technical solution to the environmental problems associated with PVC. This is especially the case in countries with advanced recycling policies, like Denmark, Germany, the Netherlands and the USA.
In reality, Greenpeace has found that PVC recycling in the main PVC consuming regions of the world amounts to less then one per cent of consumption. According to independent research, for 70 - 85% of PVC waste, recycling is not even an option for the mid to long term. This means hundreds of thousands of tonnes of PVC waste are destined to become waste in the near future creating a growing disposal problem.
The world is facing a waste crisis from PVC. Short-life PVC products, disposed of within a few years, have caused serious PVC waste problems, especially when incinerated.
The average life span of the durable products -which make up more than half of PVC consumption - is around 34 years. Durable vinyl goods produced and sold since the 1960s - when the plastic boom began - are now just starting to enter the waste stream. We are only now seeing the first stages of an impending PVC waste mountain.
There are currently over 150 million tonnes of long-life PVC materials in existence globally, used mostly in the construction sector, which will constitute this waste mountain in coming decades. Taking into account the ongoing growth in production, by the year 2005 this amount will double and the world will have to deal with approximately 300 million tonnes of PVC starting to enter the waste stream. The amount of PVC waste arising in industrialised countries is already expected to grow faster than PVC production.
So what do we do with this waste? Is there a solution? Since PVC, like most plastics, does not biodegrade quickly, three primary options exist: bury it, incinerate it or recycle it.
PVC Recycling
In the late 1980s, PVC recycling was promoted by the vinyl industry in order to make PVC more acceptable to the public and to prevent government action to limit PVC production and use. As a result, the general public and decision-makers are now accepting recycling as a technical solution to the environmental problems associated with PVC. This is especially the case in countries with advanced recycling policies, like Denmark, Germany, the Netherlands and the USA.
In reality, Greenpeace has found that PVC recycling in the main PVC consuming regions of the world amounts to less then one per cent of consumption. According to independent research, for 70 - 85% of PVC waste, recycling is not even an option for the mid to long term. This means hundreds of thousands of tonnes of PVC waste are destined to become waste in the near future creating a growing disposal problem.
|
PVC and Landfills
PVC's additives will eventually leach, posing a risk to groundwater. The bulk of petrochemical-based plastics - such as PVC, Polypropylene (PP) and polyethylene (PE) - are durable and have a long lifetime. After disposal, they do not decompose readily or quickly. Moreover, the use of many different chemical additives in some plastics results in their leaching out of landfills to contaminate soil and groundwater. This is especially true for PVC, which has the highest content of additives, most of which are hazardous to the environment. Considerable quantities of PVC are present in landfills, as a result of the disposal of municipal solid wastes (MSW), and construction and other wastes. Almost 1 million tonnes of PVC was landfilled in Europe as MSW in 1994. PVC waste from other sectors, such as agriculture, the car industry, construction and distribution is included will add considerably to this. Landfill fires become particularly toxic with PVC waste. Landfill fires are a common occurrence, with the potential to pyrolyse and combust PVC, leading to the release - either in smoke or as leachate - of a range of pollutants, including heavy metal additives and dioxins.
| |||||||